Indication :
Malignant Pleural Mesothelioma
PEMECINE® in combination with Cisplatin is indicated for the treatment of chemotherapy naïve patients with unresectable malignant pleural mesothelioma.
Non-small cell lung cancer
PEMECINE® in combination with cisplatin is indicated for the first line treatment of patients with locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. PEMECINE® is indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology in patients whose disease has not progressed immediately following platinum-based chemotherapy.
First line treatment should be platinum based with other cytotoxics chemotherapy.
PEMECINE® is indicated as a single agent for the treatment of patients with locally advanced or metastatic nonsquamous non-small cell lung cancer after prior chemotherapy (as second line). PEMECINE® is not indicated for treatment of patients with squamous non-small cell lung cancer.
Compositions :
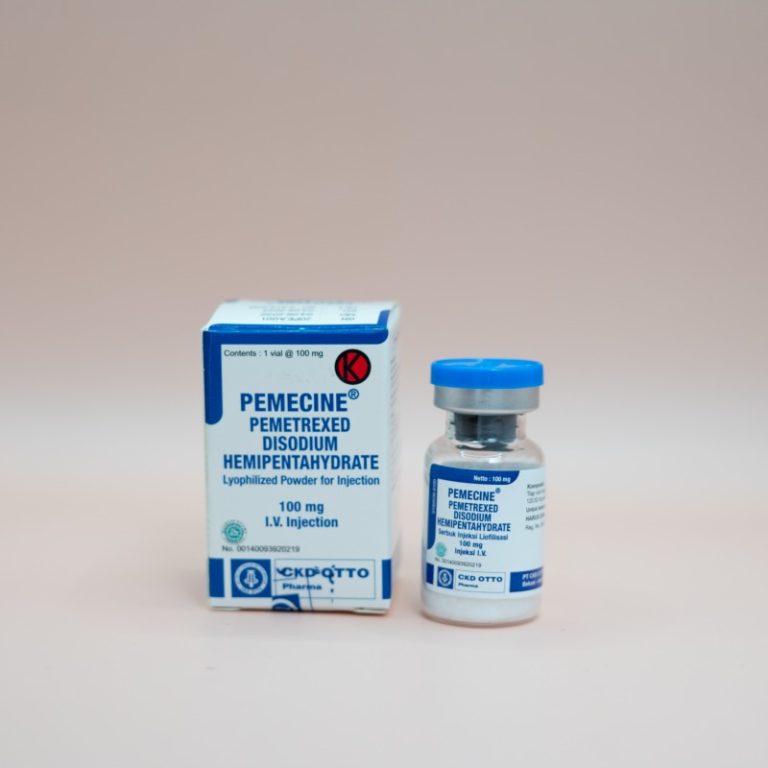
Each vial contains Pemetrexed Disodium Hemipentahydrate 120.82 mg ~ Pemetrexed 100 mg
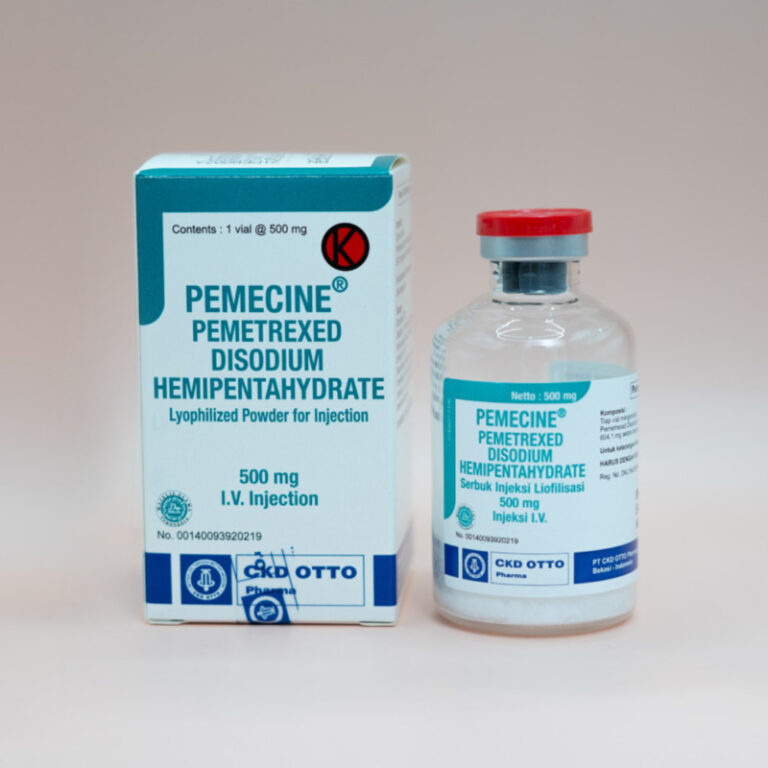
Each vial contains Pemetrexed Disodium Hemipentahydrate 604.10 mg ~ Pemetrexed 500 mg
Storage Condition :
Unopened vial : Store below 30°C.
Reconstituted and Infusion solutions : when prepared as directed, reconstituted and infusion solutions of Pemetrexed contain no antimicrobial preservatives. Chemical and physical in-use stability of reconstituted and infusion solutions of Pemetrexed were demonstrated for 24 hours at refrigerated temperature. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution / dilution has taken place in controlled and validated aseptic conditions.